Robot Nursing Device Examination Criteria
The effectiveness criteria, performance criteria, and safety criteria for the results of this research are organized as the examination criteria, and we conducted intermediate examinations and stage-gate examinations for them.
The effectiveness criteria, performance criteria, and safety criteria for the results of this research are organized as the examination criteria, and we conducted intermediate examinations and stage-gate examinations for them.
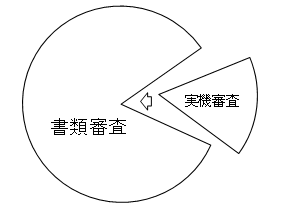
Fig. 1. The positioning of stage-gate examination document examination and actual device examination
We have made the stage-gate examination document forms
as described below.1-1 Development concept sheet (form)
2-1 Prototype development goals and issues confirmation sheet (form)
3-1 Risk assessment sheet (form)
3-2 Minimum safety verification report (form (by field))
4-1 Verification test execution plans (form)
4-2 Verification test result report sheet (form)
4-3 Verification test data, analysis results, etc. (free form)
4-4 4-4 Accident, incident, mechanical trouble, etc.
occurrence situation report (form)
5 Ethics examination related reports (applications, result reports, etc.)
6 Instruction manuals (instruction manuals assuming making products, instruction manuals for the operation of equipment used for verification tests, etc.
Note that if verification tests have not been conducted in the current fiscal year that 4-1 through 4-4 and 5 do not need to be submitted.
The latest versions of forms (as password protected compressed files) can be downloaded from the nursing robot portal site.
2. Creating Examination Criteria and Examination Methods
We have created examination criteria for the document examinations and physical examinations for the stage-gate examinations for each emphasized field. The examination criteria place an emphasis on the document examinations. As the figure shows, only for the aspects for which judgments and assessments cannot be made through the document examinations will be examinations of the actual devices be conducted.
Therefore, the examinations will be conducted in the following manner.
1) The examination documents submitted by the assisting organization will be graded by the examiners.
2) After the actual unit is examined, the same examiners will revise the document examination scores.
The examination criteria for the document examinations (and the complementary physical unit examinations) were created according to the emphasized fields for each examination item and scores assigned accordingly. The main document examinations items are listed below.
Utilization in real life according to the design concept.
Device requirement definitions deduced from the utilization in real life
Safety
Goals of the verification tests and methods
Each of the major items is to be examined by specialists in each field. The items to be examined (detailed items) within each major item are assigned points which are summed up.
In order to add the point of view of users, there will also be examinations of only the actual devices in addition to the above examination. These examinations will be conducted by examiners other than those for the document examinations, and they will examine the devices on the following items.
1. Assess the robot nursing device, not for frequently executed care tasks (supplementary care which only assists with things the users find difficult), but to see if they can provide living functions and especially if they can help to improve participatory events and activities.
2.The assessment should mainly be made from the point of view of users.
The four following items should be the examination items for the actual device examination with each item being assigned up to five points.
1.The possibility of clinical use
2.Mechanical functions
3.Clinical safety
4.Possibility of commercialization
The points given to each item require reasons for the assigned points.
5.Miscellaneous: Advice for future development
We have asked the assisting organizations to include advice or other helpful information.
The above scores should be summed up, and each assisting organization should produce their own stage-gate examination results. The document examinations (and their supplemental actual device examinations) will account for 80 out of 100 points, and the actual device examinations will account for 20 out of the 100 points.
3. Creating Actual Device Examination Methods
We made actual device examination methods for the stage-gate examinations. Actual device examinations are used to confirm and assess the performance and safety of the prototype (or final product) installed in the examination site according to the aforementioned examination criteria and examination methods. The actual device examination was held in the simulated nursing facilities in the operating test area of the Life Assisting Robot Safety Verification Center (Tsukuba City). A floor plan of the simulated nursing facilities